2023年6月,GLP-1R激动剂迎来一波小高潮,甚至出现索玛鲁肽概念股。
2023年6月26日,辉瑞宣布放弃lotiglipron的临床开发“Second GLP-1-RA candidate lotiglipron to be discontinued”。
 11月6日,Sosei Group Corporation宣布接到辉瑞公司的通知,该公司已将一种新型口服小分子 GLP-1 受体激动剂(PF-06954522)纳入1期临床试验
11月6日,Sosei Group Corporation宣布接到辉瑞公司的通知,该公司已将一种新型口服小分子 GLP-1 受体激动剂(PF-06954522)纳入1期临床试验
Tokyo, Japan and Cambridge, UK, 06 November 2023 – Sosei Group Corporation (“Sosei Heptares” or “the Company”; TSE: 4565) has been notified by Pfizer that it has entered a new oral small molecule GLP-1 receptor agonist into a Phase 1 clinical trial. PF-06954522 was discovered by Pfizer scientists during a multi-target research collaboration in which Pfizer had access to Sosei Heptares’ proprietary StaR® (stabilized receptor) technology. Pfizer recently detailed the entry of PF-06954522 into its Internal Medicine focused clinical pipeline as part of its Q3 2023 results on 31 October 2023.
笔者曾经整理过些许公开的GLP-1R小分子激动剂结构(参考链接)
而竞争对手,礼来的Orforglipron (LY3502970) 继续高歌猛进。下图,结构对比。
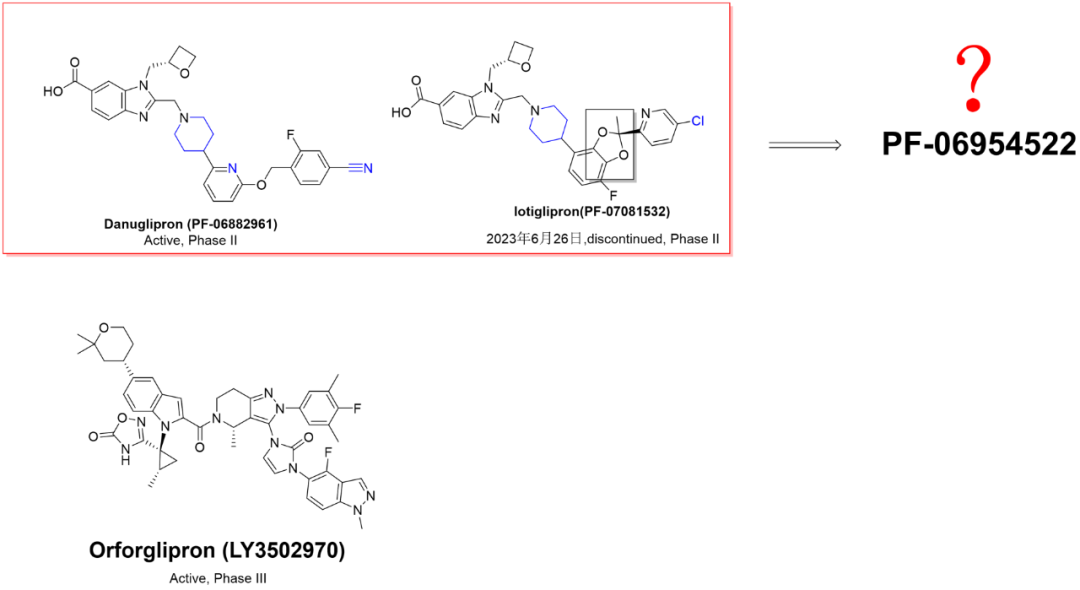
也许是辉瑞对Danuglipron (PF-06882961)的市场竞争力没有足够自信,推出了第三代(暂且定义为第三代)GLP-1R小分子激动剂。相对于众多的Follower,带头大哥转向了,Follower何去何从?
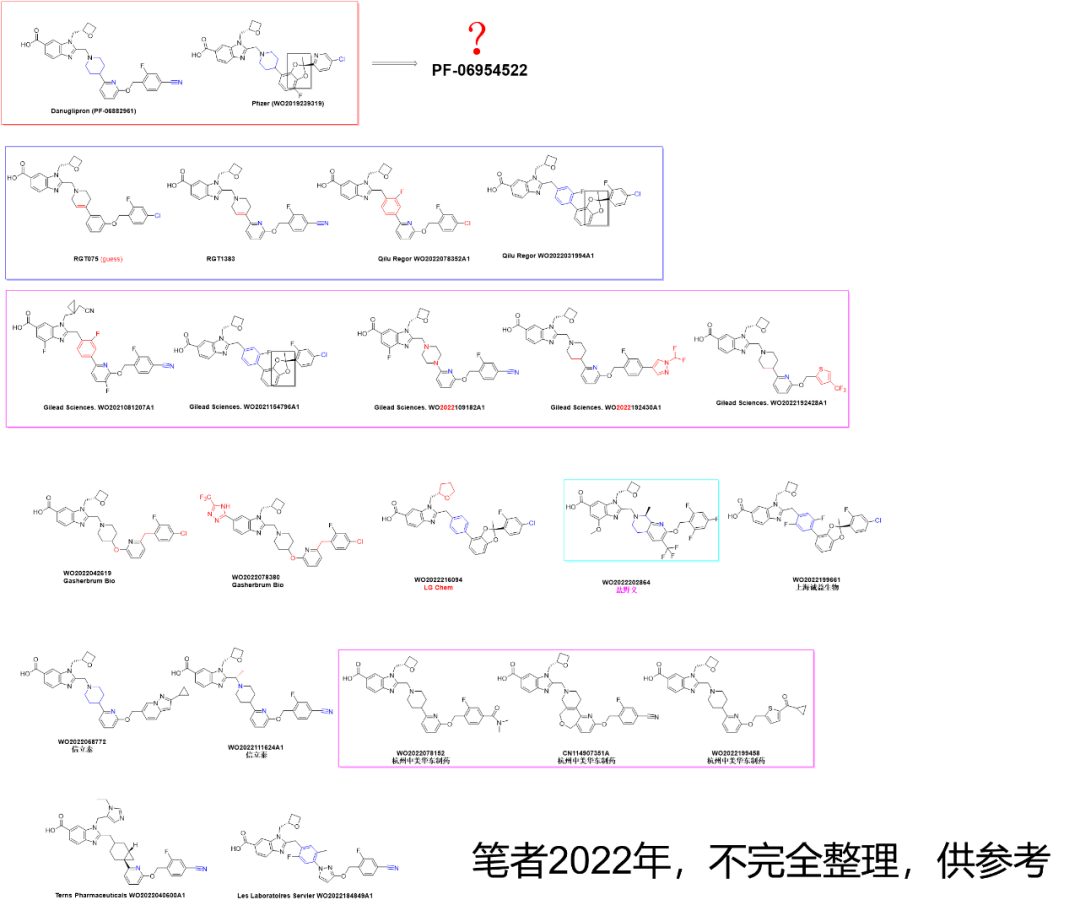
感想:还是需要差异化设计,不能“硬设计”,“硬设计”的话,带头大哥撞墙的时候,很难不撞墙。比如KRAS G12C,Amgen 的Sotorasib(AMG510)撞墙了,极度差异化的后来者Divarasib就非常惊艳,并不用担心。而Sotorasib scaffold 的 近百家Follower 就需要好好掂量掂量了。
~~~~~~~~~~~~~~~~~~~~~~~
参考笔者过往相关文章



 ufabet
มีเกมให้เลือกเล่นมากมาย: เกมเดิมพันหลากหลาย ครบทุกค่ายดัง
ufabet
มีเกมให้เลือกเล่นมากมาย: เกมเดิมพันหลากหลาย ครบทุกค่ายดัง


